For questions about financial assistance,
call 1-844-DUPIXENT, option 1 or
click to learn more
RESULTS WITH DUPIXENT (12+ YEARS)

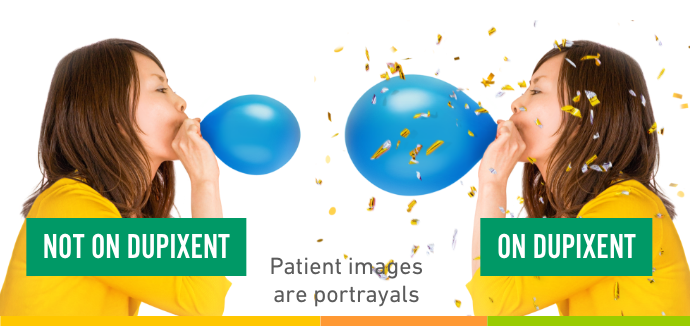
NOT ON DUPIXENT
Better breathing?
that’s a
difference
worth
celebrating.
Asthma patients on DUPIXENT
saw nearly 3x change in
breathing improvement
compared to placebo*
*In a breathing test at 1 year, 470 mL
with DUPIXENT vs 170 mL with placebo.

ON DUPIXENT
Patient images are portrayals
BENEFITS WITH EVERY BREATH
Helps improve lung
function
for better
breathing that lasts
Up to 81% fewer
asthma attacks†‡
The benefits and risks of
DUPIXENT were evaluated
in 3 clinical studies with
2,888 patients in total.
A majority of patients
reduced or eliminated
oral steroid use§
In a study of people dependent
on oral corticosteroids.
Some people felt their
asthma symptoms
improve in as little as
3 days
LONG-TERM RESULTS WITH DUPIXENT
An additional continuation study up to 3 years analyzed the safety and efficacy of 2,282 eligible patients
who completed a parent study. Patients received 300 mg of DUPIXENT every 2 weeks.
In this study, all researchers knew participants received DUPIXENT, which could have influenced the results. Since participants completed a previous
study, results might have favored those who already responded well to DUPIXENT. The parent trials were designed to avoid these biases.
DUPIXENT demonstrated
lasting breathing
improvement
up to 3 years (447 patients)
![]()
experienced zero
asthma attacks
in the 3rd year of
treatment (816 patients)
SAFETY INFORMATION FOR DUPIXENT
The most common side effects
The risks of DUPIXENT were evaluated in 2 clinical studies involving 2,359 patients in total,
ages 12 and older. The side effects below occurred more with DUPIXENT than placebo.
INJECTION SITE REACTIONS¶
| DUPIXENT 200 mg (779 patients) |
14% |
| DUPIXENT 300 mg (788 patients) |
18% |
| Placebo (792 patients) |
6% |
PAIN IN THE
THROAT (OROPHARYNGEAL PAIN)
| DUPIXENT 200 mg (779 patients) |
2% |
| DUPIXENT 300 mg (788 patients) |
2% |
| Placebo (792 patients) |
1% |
HIGH COUNT OF A CERTAIN WHITE BLOOD CELL (EOSINOPHILIA)
| DUPIXENT 200 mg (779 patients) |
2% |
| DUPIXENT 300 mg (788 patients) |
2% |
| Placebo (792 patients) |
<1% |
View the possible side effects of DUPIXENT in patients with moderate-to-severe eosinophilic or oral corticosteroid–dependent asthma.
¶Injection site reactions includes injection site reaction, pain, bruising, and swelling. Talk to your doctor about this side effect. If experienced, try a cold compress.
For both adults and children, the safety profile of DUPIXENT through Week 52 was generally consistent with the safety profile observed at Week 24.
TAKE CHARGE OF THE CONVERSATION WITH YOUR DOCTOR
When my doctor suggested DUPIXENT, I was
open to it. We had a candid conversation. I asked
a lot of questions.
Rachel,
Real patient. Individual results may vary.
Prepare for your next doctor’s visit with our discussion guide.
start your guideDo you have a doctor who treats your asthma?
Select the best option to receive information to address your specific needs.
Great! Consider discussing DUPIXENT as a treatment option with your specialist. Let us help you create a personalized doctor discussion guide to bring to your next appointment.
Working with an asthma specialist can help you or your loved one create a personalized treatment plan and discuss medicines like DUPIXENT.
It’s important to work with a specialist for thorough asthma management. Consider setting up an appointment with an allergist or pulmonologist.
Frequently Asked Questions
The average age of the patients in the study was 49 years old. These patients averaged 2 asthma attacks in the previous year, and 64% were on high-dose inhaled corticosteroids.
In clinical trials, the impact of DUPIXENT on lung function was studied in patients 6 to 11 years of age and patients 12 years of age and older. Patients in each age group saw improved lung function in as little as 2 weeks.
Learn how DUPIXENT helped treat children 6 to 11 years old with their moderate-to-severe asthma.
DUPIXENT can cause serious side effects, including:
- Allergic reactions. DUPIXENT can cause allergic reactions, including skin reactions, that can sometimes be severe. Stop using DUPIXENT and tell your healthcare provider or get emergency help right away if you get any of the following signs or symptoms:
- Inflammation of your blood vessels. Rarely, this can happen in people with asthma who receive DUPIXENT. This may happen in people who also take a steroid medicine by mouth that is being stopped or the dose is being lowered. Tell your healthcare provider right away if you get:
- rash
- worsening shortness of breath
- persistent fever
- chest pain
- brown or dark colored urine
- a feeling of pins and needles or
numbness of your arms or legs
- Psoriasis. This can happen in people with asthma who receive DUPIXENT. Tell your healthcare provider about any new skin symptoms. Your healthcare provider may send you to a dermatologist for an examination if needed.
- Joint aches and pain. Joint aches and pain can happen in people who use DUPIXENT. Some people have had trouble walking or moving due to their joint symptoms, and in some cases needed to be hospitalized. Tell your healthcare provider about any new or worsening joint symptoms. Your healthcare provider may stop DUPIXENT if you develop joint symptoms.
The most common side effects of DUPIXENT include:
- injection site reactions
- pain in the throat (oropharyngeal pain)
- high count of a certain white blood cell (eosinophilia)
- parasitic (helminth) infections
Do not stop taking your corticosteroid medicines unless instructed by your healthcare provider. This may cause other symptoms that were controlled by the corticosteroid medicine to come back.