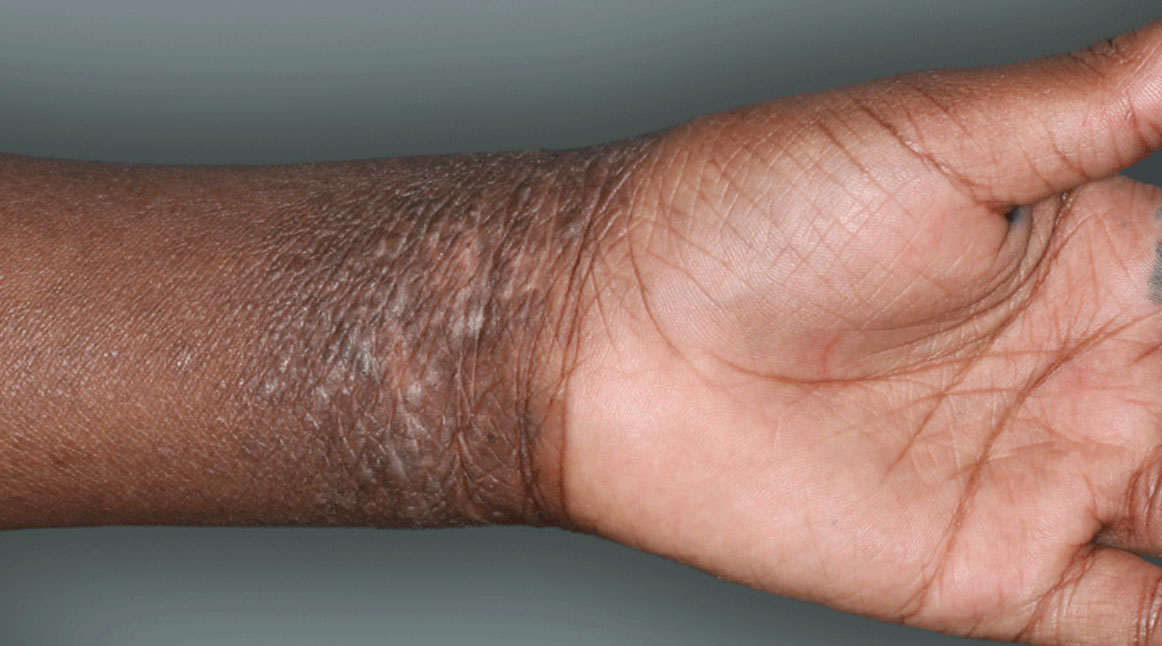
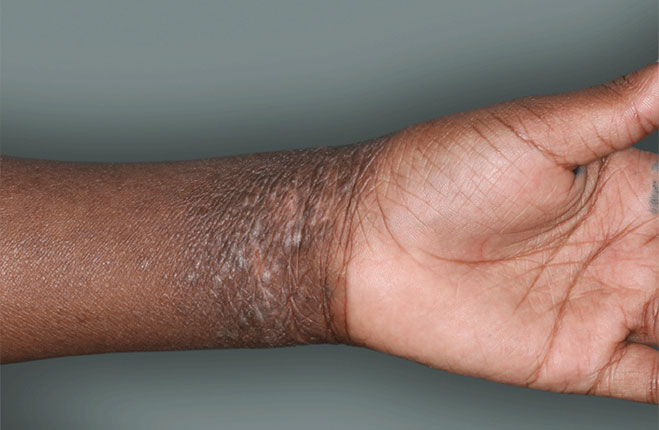
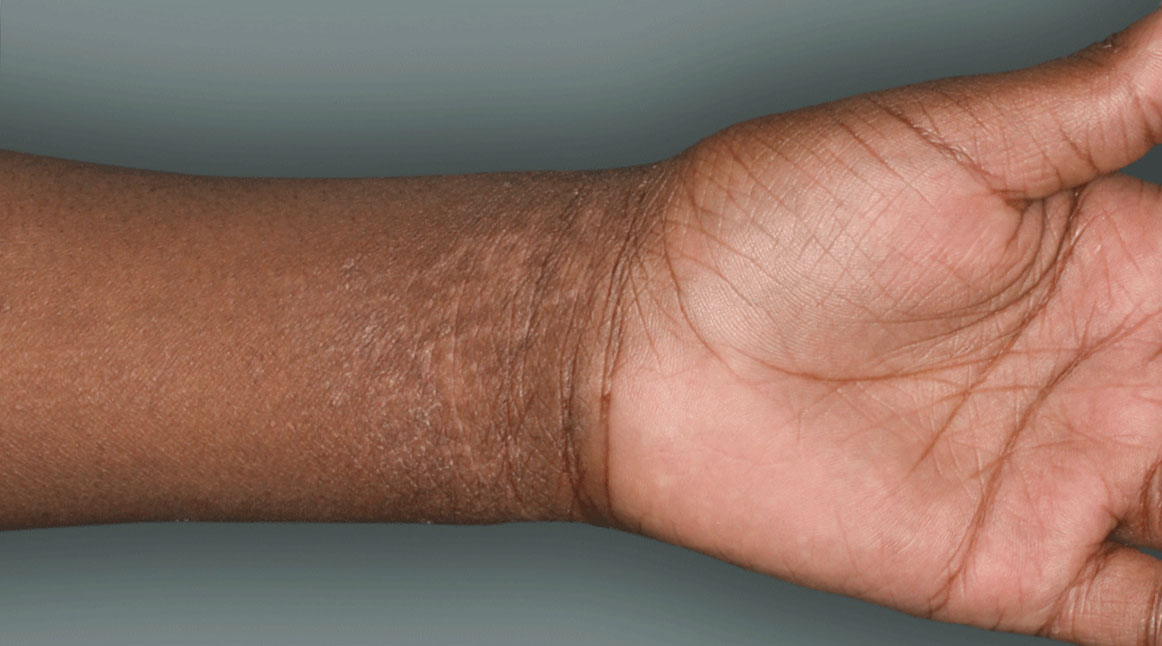
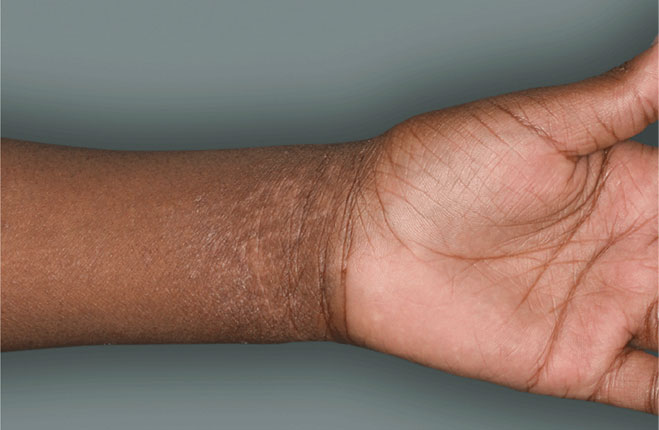
I grew up in a very small town—one stop light, if you blink you might miss it. Very quaint. The small-town environment lends to the type of work that I do, more one-on-one with our patients.
My name is Shari and I’m a registered nurse with DUPIXENT MyWay.
I chose to be a nurse because I wanted to help people, and I believe that people should be in service to others.
Being a nurse for DUPIXENT MyWay is very rewarding. I really enjoy the patient interaction. I’m ready to make a difference. I’m ready to help our patients to have the confidence to proceed with their journey.
Our nurses work remotely from our homes. So, we step into our offices, we’re going into a nice relaxing environment.
When our patients call in, they get to speak to a real person, a experienced clinician who can provide them support along their journey.
We provide general product support and education, as well as supplemental injection training, and injection and refill reminder calls.
Every day is different depending on the type of calls that we have. Some of the common questions we get: “How much is it going to cost me?” “When will I get started?” “How do I take my DUPIXENT injections when I’m traveling?”
We have multiple resources available for patients. We have the ability to send out package inserts that include all the important safety information for DUPIXENT. For patients wanting a copay card, they can access that by visiting our product website at DUPIXENT.com.
One of my favorite parts of providing nursing care to our patients is being able to walk them through their journey, hold their hand through the process, just to give them confidence along the way and we always want them to know that they have our support.
LAURIE, Field Nurse
I’m Laurie. I’m a registered nurse with DUPIXENT MyWay. I’ve been with DUPIXENT MyWay since the very beginning.
I give supplemental injection training to the patient and the patient’s caregiver.
When I was very young, I knew that I wanted to be a nurse. I wanted to go out and make a difference and help people.
When I get the order for the patient, I get really excited. I travel to see the patients—sometimes for hours. I make sure that I gather all of the things that I may need to help the experienced person, and then the person that’s never even touched a needle.
I think it’s very important for me as a nurse to go and teach these patients. Not only to teach them how to give themselves the medicine, but also to just come and give them encouragement, and show them kindness and patience.
When I go to see the patient, I can’t wait to travel, no matter how far it is. I have a training kit that has a training syringe in it. And that helps us so that we can practice before we do the real thing.
I can’t wait to go and meet them, and see where they are in their life, and be excited for them.
I help them to relax. Sometimes they can just take a deep breath, we’ll go slow, think about their favorite place, think about something that reminds them and makes them happy and calms them down.
I went to this patient’s house and he seemed very standoffish. You could tell that the patient was just not comfortable. He was anxious, he wouldn’t give me eye contact. So, I asked the parents, “would it be OK if I just come back the next day?"
I think it’s very important to just be patient. Make it into their routine and let them go at their own pace. We have time. They have set aside this time for us to learn. And for me to teach.
Sometimes at the end of the training, I’ll get some questions about, “You know, we did great today, but what else? What if when you leave, I don’t know what to do and it’s time for me to give myself my injection again?” I can refer the patient to the DUPIXENT website for more resources and there’s also a Nurse Educator phone number.
I like to go out and do this job. It’s just really neat. It’s neat to go in and get to know people.
DUP.23.04.0076
Transcript
DUPIXENT® (dupilumab)—a medicine with special storage requirements—is what’s known as a specialty medicine.
That means it may be delivered to you by a specialty pharmacy instead of your local pharmacy.
That also means you can expect the process for getting your prescription filled to be different from other medicines you may pick up from your local pharmacy.
It will go something like this:
First, your doctor writes a prescription for DUPIXENT. Be sure to ask your doctor about enrolling in DUPIXENT MyWay®, which can provide additional support for you. You or your doctor can download the enrollment form on DUPIXENT.com or call 1-844-DUPIXENT, option 1 to enroll.
Next, your prescription may have to be authorized by insurance. This is called prior authorization and is common for specialty medicines.
Your insurance company will work with your doctor to get any additional medical information they need.
It might feel like this part takes a while, but hang in there.
If you have enrolled in DUPIXENT MyWay, you will receive a welcome call from a Nurse Educator while your insurance benefits are being confirmed. Once DUPIXENT is approved by your insurer, a specialty pharmacy works with you to schedule the shipments of DUPIXENT to your home or other preferred location, so be sure to answer their calls to prevent delays.
They will not ship your medicine without first confirming delivery details with you.
And while everyone’s working through the details, look to DUPIXENT MyWay for additional support.
You can connect with DUPIXENT MyWay Nurse Educators by phone to receive supplemental injection training, help scheduling deliveries and prescription refills, or help navigating financial support options, such as copay assistance.
As a reminder, with all of these folks helping to get you off to good start with DUPIXENT, you may receive phone calls from your doctor’s office, specialty pharmacy, and a DUPIXENT MyWay Nurse Educator.
Remember to quickly respond to these calls to avoid delays in receiving DUPIXENT. So, now you know what to expect from prescription to delivery.
For more information on how to properly store DUPIXENT after delivery, please review the DUPIXENT Instructions for Use at DUPIXENT.com.
Transcript
VO: DUPIXENT is a prescription medicine used:
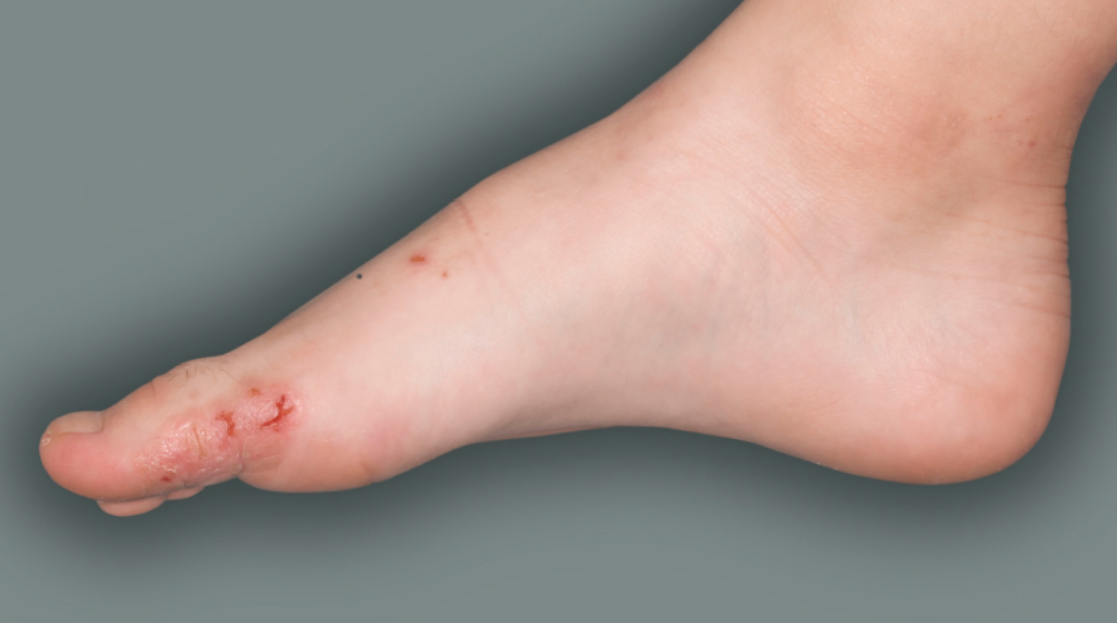
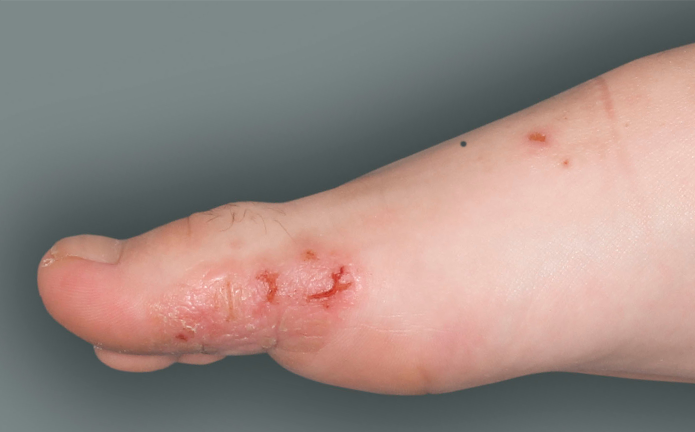
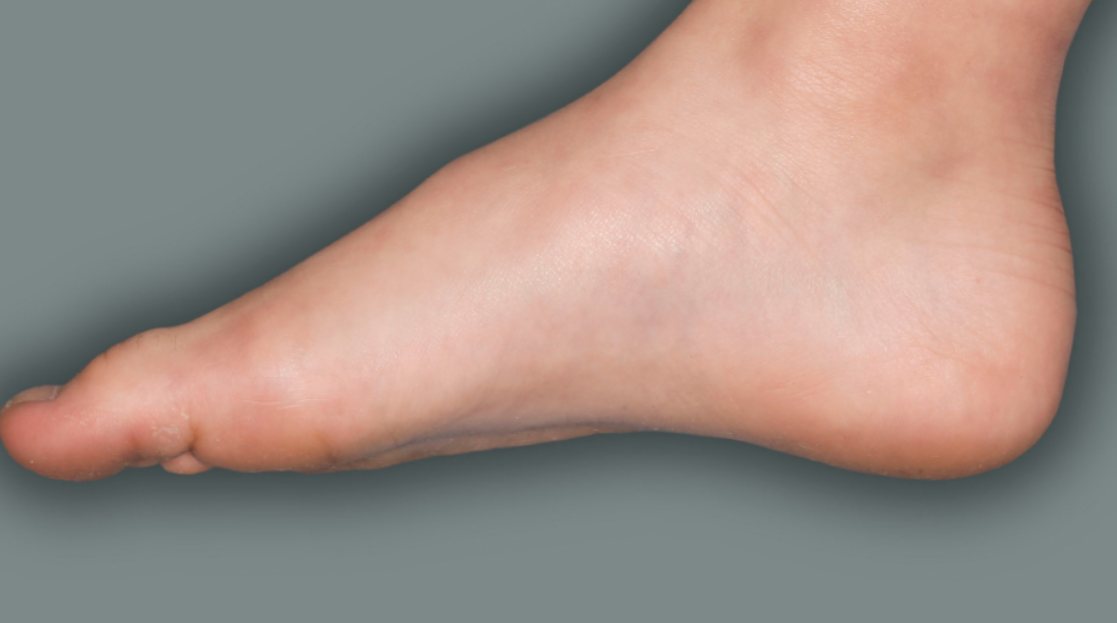
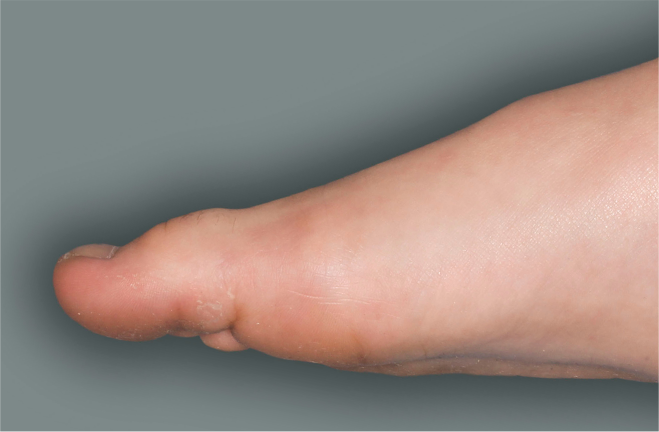
- to treat people aged 6 years and older with moderate-to-severe atopic dermatitis (eczema) that is not well controlled with prescription therapies used on the skin (topical), or who cannot use topical therapies. DUPIXENT can be used with or without topical corticosteroids. It is not known if DUPIXENT is safe and effective in children with atopic dermatitis under 6 years of age.
- with other asthma medicines for the maintenance treatment of moderate-to-severe eosinophilic or oral steroid dependent asthma in people aged 12 years and older whose asthma is not controlled with their current asthma medicines. DUPIXENT helps prevent severe asthma attacks (exacerbations) and can improve your breathing. DUPIXENT may also help reduce the amount of oral corticosteroids you need while preventing severe asthma attacks and improving your breathing. DUPIXENT is not used to treat sudden breathing problems. It is not known if DUPIXENT is safe and effective in children with
- with other medicines for the maintenance treatment of chronic rhinosinusitis with nasal polyposis (CRSwNP) in adults whose disease is not controlled. It is not known if DUPIXENT is safe and effective in children with chronic rhinosinusitis with nasal polyposis under 18 years of age.
Important Safety Information
Do not use if you are allergic to dupilumab or to any of the ingredients in DUPIXENT®.
Please see additional Important Safety Information throughout this video and adjacent links for full Prescribing Information asthma under 12 years of age.
Before starting DUPIXENT, you should talk to your doctor about all the medical conditions you have and medications you are taking.
You and your doctor should also discuss the potential benefits and risks of treatment with DUPIXENT including the most common side effects such as injection site reactions, and some serious side effects such as allergic reactions including anaphylaxis, eye problems and inflammation of your blood vessels.
JENNIFER: If you’re thinking about trying DUPIXENT, I would say, talk to your doctor, get connected with DUPIXENT MyWay, and make sure you’ve got a support system out there cheering you on. manage the disease.
RACHEL: I highly recommend getting a good physician that you can talk to that you trust, and have that open communication. Develop a good treatment plan together and advocate for yourself.
KRISTY: Know that there is DUPIXENT MyWay. There's always someone there to support you and that they're on your side. If any concerns arise or you have any problems, that there will always be someone there to help you.
CHLOE: It was so nice knowing that I could call that number anytime to talk to a nurse or whoever to give me more information to find out more about DUPIXENT, whether it was payment or how to inject or anything like that.
SUE: I don’t even think about giving myself an injection anymore. It’s just like putting on makeup or brushing your hair. It’s just there.
But everybody’s different. I know it can be kind of nerve-wracking, it’s so worth it.
JACQUE: There's help out there for you now. The advice I would give someone starting DUPIXENT is to ask all the questions that you have. Talk to your doctor.
Be optimistic. Be persistent and look forward to the possibilities that lie ahead of you with DUPIXENT.
VO: Do not use if you are allergic to dupilumab or to any of the ingredients in DUPIXENT®.
Before using DUPIXENT, tell your healthcare provider about all your medical conditions, including if you:
- have eye problems
- have a parasitic (helminth) infection
- are scheduled to receive any vaccinations. You should not receive a “live vaccine” if you are treated with DUPIXENT.
Transcript
Let’s take a second to get situated before we begin our breathing exercise.
Find a spot where you can be still and quiet. Get into a comfortable position. This can be seated, standing, or laying down. Pause for just a moment before you begin.
Now, close your eyes and settle your mind.
Focus on your body relaxing as you settle into position. Relax your muscles; start with your toes, work upward to your arms and then your head. Transition your focus to your breath.
Slowly take a deep breath. Let your stomach expand [pause], and now your rib cage, and your chest. Pause for just a moment.
Exhale at the same pace as the inhale. Allow the breath to come steadily out of your nose until your lungs are completely empty.
Pay attention when you breathe in. First your stomach rises, then your rib cage, finally your chest. Notice each of them fall as you breathe out: chest, rib cage, stomach.
Feel your body respond as you continue to breathe.
This can be done for as long as you like. You can repeat this any time you are feeling anxious to help calm yourself.
VO:
DUPIXENT is a prescription medicine used to treat adults and children 6 months of age and older with moderate-to-severe eczema (atopic dermatitis or AD) that is not well controlled with prescription therapies used on the skin (topical), or who cannot use topical therapies. DUPIXENT can be used with or without topical corticosteroids. It is not known if DUPIXENT is safe and effective in children with atopic dermatitis under 6 months of age.
Important Safety Information
Do not use if you are allergic to dupilumab or to any of the ingredients in DUPIXENT®.
Please see additional Important Safety Information throughout this video and adjacent links for full Prescribing Information.
Individual results with DUPIXENT may vary.
Sponsored by Regeneron and Sanofi.
ORE:
I like when my dad does my injection for me. He was trained by my doctor.
YETUNDE:
Ore’s dermatologist trained us on how to do the injection under the skin, and then when we contacted DUPIXENT MyWay, they sent a nurse to the house to give additional training to make sure that we were comfortable giving the injection.
ORE:
I feel like one day I will do it myself, but I think right now assisted injection is better.
YETUNDE:
I do remember her dad asking, “Are you sure that you’re comfortable with this,” it being an injection under the skin, and she was. I’m the one that cringes with needles, but she does great.
ORE:
I see the injection as something that I just have to do, and it doesn’t freak me out as much as it did in the beginning. I don’t really get scared anymore.